Understanding the Science and the Process of Developing an Immunity
The global population is still ongoing due to the COVID-19 pandemic, and this makes the role of viral vaccine manufacturing crucial more than ever before. Vaccines prevent millions of deaths annually, and when the viruses change forms or debut, the process of creating vaccines starts.
But yet it raises another question, how exactly are viral vaccines manufactured? The production of the virus vaccine presents some difficulties to the scientists and manufacturers in the following ways. So, here we are in the amazing, intricate and essential world of viral vaccines production now.
What is Viral Vaccine Manufacturing?
Viral vaccine manufacturing is a large-scale virus vaccine production process aimed at developing immunity against viral diseases. While Antibiotics are used to cure diseases, vaccines on the other hand, are used to prevent diseases. They operate by exposing the immune system to a harmless part of a virus or closely related virus and thereby the immune system knows how to defend itself against a real virus attack.
Introduction to the Viral Vaccine Manufacturing
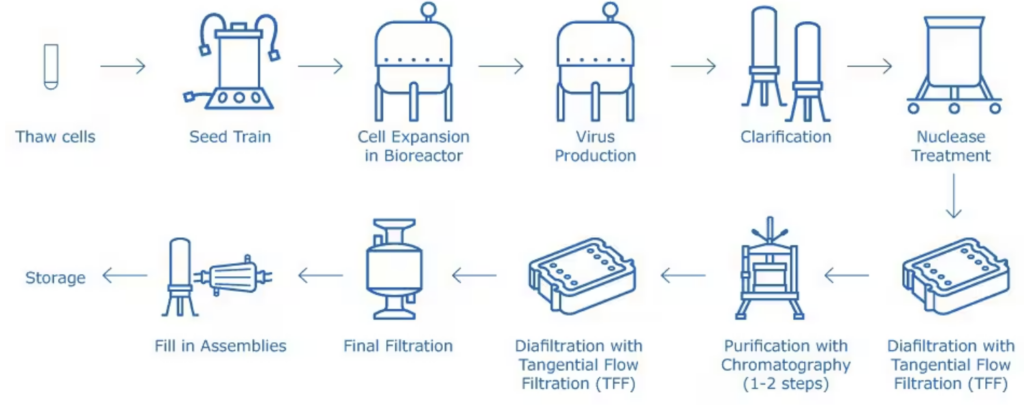
To produce the vaccine, one has to go through a number of phases to create a safe one that has met all the scientific, legal, and technological requirements. Here are the major stages:
1. Virus Selection & Isolation
First of all, the virus strain to be targeted is determined by scientists. This involves:
- Analyzing epidemiological data
- This is due to the fact that in order to be effective enough to cause illness in patients, the strain used has got to be potent enough to be taken through the necessary processes of modification or inactivation.
2. Cell Culture Preparation
Since viruses can only replicate in host cells, the appropriate cell lines such as Vero, CHO, or MDCK are used to propagate the virus. These cell cultures are used for the purpose of virus replication.
3. Virus Cultivation
The selected virus is then added to the cell culture system, thus it penetrates the cells and replicates. Some of the factors like temperature, pH and nutrient concentrations are regulated deliberately.
4. Harvesting
After sufficient growth, the virus is harvested from culture by methodically replacing the nutritive medium in the culture with a schematic one. This step may involve:
- Centrifugación
- Filtración
- Ultrafiltration
5. Inactivation or Attenuation
This step is critical depending on the type of vaccine that is being used:
- In inactivated vaccines, the virus is rendered noninfectious by heat or chemical treatment such as treatment with formalin.
- In live-attenuated vaccines the virus is slightly altered so that it can reproduce but it is not virulent.
6. Purification
Contaminants such as cell debris and other elements in the media are eliminated. Techniques include:
- Chromatography
- Ultracentrifugation
- Precipitation
7. Formulation
After purification the virus or viral component is mixed with diluents and stabilisers and adjuvants if necessary.
8. Filling and Packaging
Sterile conditions are mandatory. The vaccine is then placed into vials or syringes, then labeled, closed and prepared for dispatch.
9. Quality Control and Testing
In order to maintain the name of the product and customer satisfaction, safety, potency, and purity, as well as the quality check, a wide range of tests are conducted at every step. Usually, agencies such as the FDA, EMA, and WHO set high standards in the regulation of vaccines.
Techniques used in Viral Vaccine Manufacturing

There isn’t a one-size-fits-all approach. The procedure is used based on characteristics of the virus and the type of the intended vaccine.
Live-Attenuated Vaccines
- Use weakened live viruses
- Examples: MMR, Yellow Fever
Inactivated Vaccines
- Use killed viruses
- Examples: Hepatitis A, Rabies
Subunit and Recombinant Vaccines
- Use viral proteins or pieces
- Examples: Hepatitis B, HPV
Viral Vector Vaccines
- Treat the virus as non-pathogenic so that the genetic material can be delivered into the target cell.
- Examples: Johnson & Johnson’s COVID-19 vaccine
mRNA Vaccines
- form viral proteins in the mRNA that will be transported through lipid nanoparticles
- Examples: Pfizer-BioNTech, Moderna
Challenges in viral vaccine development
However, there are some problems that remain unresolved to date regarding development of viral vaccines:
1. Virus Mutation
Influenza and SARS-CoV-2 that affect humans are known to have high mutation rates. Such changes may lower the efficacy of the vaccine and may have to be updated from time to time.
2. Cultivation Complexity
Certain viruses cannot be cultured easily or cannot be cultured at all in the classic cell cultures.
3. Regulatory Hurdles
Clinical trials of drugs require phases that have to be completed in a number of years and at a great cost.
4. Cold Chain Requirements
Most vaccines are sensitive to temperature changes and this is even more so with the new mRNA vaccines complicating the supply chain.
5. Scale-Up Difficulties
It is not easy to scale up a process from laboratory-scale to pilot scale to industrial scale. The major issues for concern include consistency, sterility, and cost.
6. Public Trust & Misinformation
This may be so even with the most effective vaccine in the world if people are adamant on not taking it. The three core values in this regard include communication, and education as well as transparency.
Innovations Shaping the Future

Biotechnology today is progressing continuously, and it has revolutionized the process of viral vaccines manufacturing with better technologies.
- CRISPR: Precision gene editing for better viral vectors
- AI & Machine Learning: Predicting antigenic targets and vaccine responses
- Biología sintética: Designing custom antigens
- Nanotechnology: Enhancing delivery systems and immunogenicity
Occasionally termed as ‘endless manufacturing’ or ‘stream manufacturing,’ continuous virus vaccine production process is the process of manufacturing products in a non-stop fashion, thereby helping organizations to cut down the amount of time spent on production and control the wastage of resources.
Conclusión
The process of viral vaccine manufacturing is a fusion of science, engineering, and strict regulation. From initially defining a viral strain to distributing billions of doses over the world there are many challenges in viral vaccine development. Nevertheless, each achievement not only assures the elimination of fatal viral diseases but also warms people up for the next one.
The emergence of new viruses will require the continuation of a good global collaboration and investment in vaccines. Explaining how vaccines are manufactured cannot be considered a topic exclusive to the scientific community only anymore. It is an issue that every citizen of the world should concern himself or herself with.
A major question that has been posed by the current health change is whether the world is prepared for the next pandemic? It is now appropriate that we ensure our vaccines are. Do check out our more blogs!
Preguntas frecuentes
Q1. What are the stages of viral vaccines manufacturing?
Some of the common steps involved in viral vaccines production include virus growth and recovery, virus purification, virus inactivation and most important for live attenuated vaccines, virus attenuation, formulation with stabilizers or adjuvants and final filling and sealing. Policies and standards compliance involves testing to ensure that the vaccine is safe throughout the whole process before it is released into the market with the right quality and standard as required by health standards.
Q2. How does a virus incubation period relate to the contagious period?
The incubation period is the time between the contact with the virus and the development of symptoms, while the contagious period is the time during which the infected person is transmitting the virus to the others. Depending on the nature of the virus, it will have a preferred operating system. Some need live cells to replicate while others may use eggs most especially the traditional flu vaccines.
Q3. Why is purification crucial in viral vaccine manufacturing?
This is important because impurities that may be present during virus vaccine production process like cellular debris, culture media or other constituents may affect safety as well as efficiency of the vaccine. Chromatography, ultra – filtration and centrifugation procedures are often employed in order to check if the vaccine is of high purity or not.
Q4. What are currently various difficulties that viral vaccine developers have to deal with?
Some of these are:
- A virus that changes periodically and needs to be reconstituted periodically (for example influenza)
- Some viruses are difficult to grow in culture media and in some cases, it is virtually impossible to do so.
- Maintaining organisational safety and large-scale production is also known as achieving organisational reliability.
- High costs and long timelines for R&D
- Regulatory complexities and compliance with international guidelines
Q5. How is quality control managed during manufacture of viral vaccines?
Quality check is conducted every step of the process, right from the inputs used as well as the final products that are manufactured. This encompasses sterility tests, tests to determine the strength or potency of productions, safety measures as well as stability tests. Regulatory authorities like the WHO, FDA, and EMA set strict guidelines for vaccine quality.
Q6. Is it true that the developments in biotechnology have enabled it to reduce or eradicate the need to employ the products derived from animals in the creation of viral vaccines?
This shift is beneficial for the ethical and minimizes the possibilities of contamination originating from animal-derived products.
Q7. Have viral vaccine technologies played a crucial part in the COVID-19 pandemic? Technologies like viral vector platforms, mRNA-based vaccines helped to create safe and effective vaccines at a faster rate compared to the past. In a way, these platforms were not your run of the mill viral vaccine manufacturing since there was a need to create immunity fast and on a large scale.