Have you ever thought about how some of these medicines are effective by simply halting the adverse reactions in the body? Or why does food spoilage decrease when it is placed in the refrigerator? The answer is the enzyme inactivation which is widely involved in biology, medicine, and industry.
Actually enzymes are proteins that act as biological catalysts that increase the rate at which chemical reactions take place and in some instances, their activity requires regulation. Enzyme inactivation can thus can be thought of as enzyme inhibitors or inhibitors of enzymes, irrespective of the methods employed in enzyme inactivation , knowledge of how enzymes are inactivated plays a vital role in the development of drugs, food preservation and the control of diseases.
In this article, the reader will also learn what enzyme inactivation is, how it occurs, and its significance in different areas.
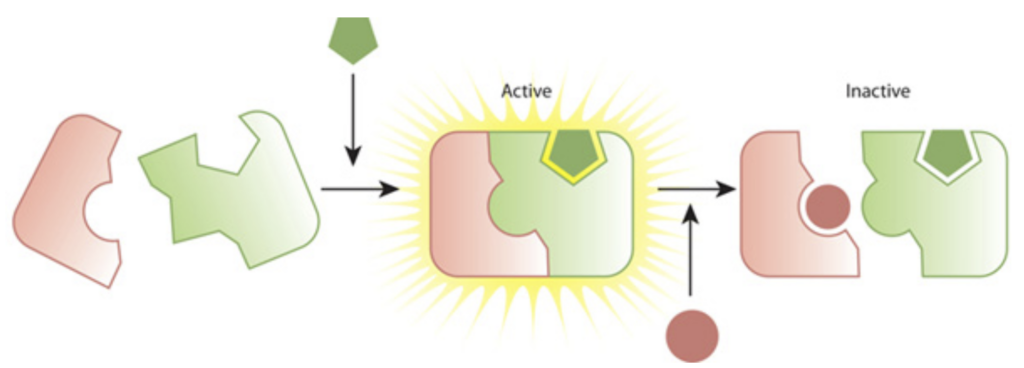
What Is Enzyme Inactivation?
Enzyme inactivation means the process in which an enzyme, which has catalytic properties for a certain reaction, fails to catalyse the particular reaction. This may be either short-term or long-term based on the cause behind it.
Key Features of Enzyme Inactivation:
- Loss of enzyme activity due to structural changes.
- Can be non-competitive or competitive depending on inhibition nature.
- Occur both as naturally occurring substances and artificially produced compounds that have impact on metabolism, medicine, and biotechnology.
For instance, ever considered a situation where your enzymes in the gastrointestinal tract cease to function? The body will be unable to digest food for energy. That is why the inactivation of enzymes is an equally important and tightly controlled occurrence.
Causes of Enzyme Inactivation
There are different causes that may result to enzyme inactivation as explained below:
1. Temperature Changes
Enzymes are known to act at optimal temperatures and hence they have different temperatures which are most appropriate to act. Heat can inactivate enzymes that are involved in the improvement of its structure and formation of new better structures. This is why cooking food kills bacteria; the enzymes are denatured. On the other hand, storage of food reduces the rate of activities carried by the enzymes which are responsible for the spoiling of food items.
2. pH Variations
Enzymes also have an optimal pH range in which they perform their operations efficiently. High acidity or alkalinity has an impact on the shape of the enzyme hence its ability to function or not at all. For instance, pepsin, which is a digestive enzyme in the stomach, is active in an acidic environment while amylase in the saliva is active in a neutral environment.
3. Chemical Agents
Some substances which include heavy metals, detergents and solvents act by altering the structure of enzymes and hence bond in irreversible enzyme inhibition . Most of the poisons or toxins are known to work by irreversibly going for the enzymes’ activity. For instance, cyanide inhibits cytochrome c oxidase that forms an important enzyme in cellular respiration hence leading to quick cell death.
4. Substrate or Product Accumulation
Substrate and product appear to inhibit the enzyme activity If their concentrations are high, the reaction rate is slowed down or ceases completely. This is a familiar form of feedback mechanism which helps to eliminate the build up of some substances in metabolic processes.
5. Genetic Mutations
Alterations in genes that code for enzymes may lead to formation of non-functional enzymes causing diseases due to lack of enzymes. Some metabolic disorders include phenylketonuria (PKU) and this can be categorized as enzymopathies because they originate from nonfunctional enzymes that need to be managed by diet or medication.
Enzyme Inhibition Mechanisms
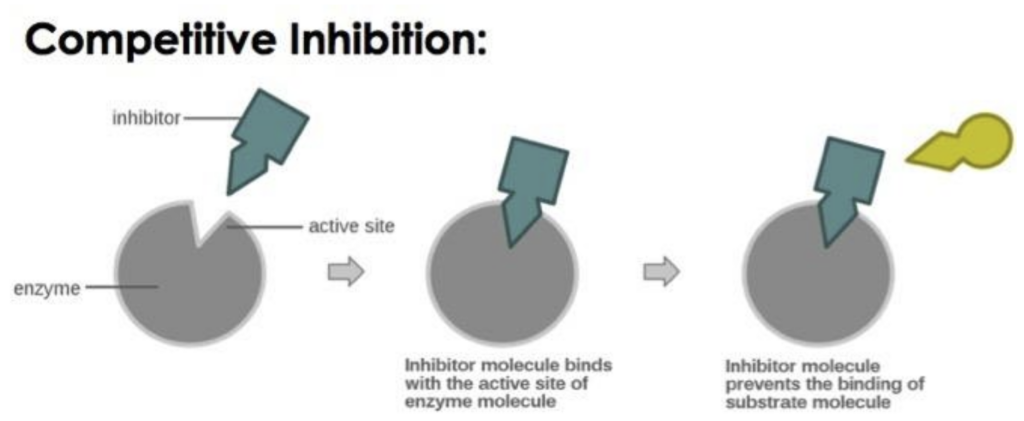
These mechanisms are aimed at regulating the activity of enzymes in the body either temporarily or permanently. There are two main types:
1. Reversible Enzyme Inhibition
In this type of enzyme inhibition mechanism , the enzyme activity can always be regained once the inhibitor in use is removed. There are three types:
- Competitive inhibition: An inhibitor directly binds with the substrate and has a similar shape to the substrate to fit into the active site of the enzyme. An example of drugs that inhibit enzyme activity is when the drugs bind to the active sites because they are similar in structure to the substances that ordinarily bind there. A good example is methotrexate that is known to work by stopping the action of dihydrofolate reductase hence slowing down the growth of cancer cells.
- Non-Competitive Inhibition: The inhibitor does not bind to the active site but it interacts with other sites on the enzyme thus alters its shape in a way that decreases its activity. This is widely observed in metabolic regulation processes.
In this case the inhibitor only binds to the enzyme-substrate complex and the reaction never comes to an end. This is used in some drugs to increase selectivity towards the target Since some drugs contain a number of target molecules, this mechanism is used in order to increase the selectivity of the drug for a particular target.
2. Irreversible Enzyme Inhibition
In this form of irreversible enzyme inhibition the inhibitor forms a covalent bond with the enzyme and this is a permanent bond that denies the enzyme its functionality. This is seen in:
- Covalent binding: It directly attaches to the enzyme involving a chemical connection which makes the inhibitor permanently unbindable. For instance, nerve gases interfere with the function of enzymes in the nervous system.
- Inactivation of the active site: Many inhibitors weaken or alter the shape of the active site so that the enzyme cannot recognize the substrate.
You would not believe that many antibiotics are operating through mechanisms of irreversible enzyme inhibition , thus bacteria can not grow.
Applications of Enzyme Inactivation
1. Pharmaceutical Industry
Enzyme inhibition mechanism is a common approach used in drug design involving disease treatment. For instance, statins cut down on enzymes that synthesize cholesterol and hence, decrease the possibilities of heart diseases. Similarly, protease inhibitors are used as an HIV medication too as they prevent the replication of the virus.
2. Food Preservation
Heat and chemical preservatives kill these enzymes within the foods therefore reducing food spoilage period. In the case of pasteurization, the utilization of enzymes to inactivate bacteria in milk makes it safe to consume. Besides, before freezing, the vegetables must be blanched to reduce enzyme activity that leads to decay.
3. Agriculture and Pest Control
The use of the pesticides involves the immobilization of specific enzymes in insects and weeds to prevent them from presenting a threat to crops. For instance, the organophosphate agents deny acetylcholinesterase, which results in paralysis of insects.
4. Metabolic Disorders
Some genetic disorders like phenylketonuria require the use of enzymes which are not produced properly thus necessitating a procedure to neutralize them. People are working in developing enzyme replacement therapies to treat affected individuals with enzyme deficiencies.
5. Industrial and Biotechnology Applications
In current biotechnology, there is a necessity to control enzyme inactivation in order to stop the undesired reactions. For instance, enzymes used in brewing and dairy industries are deliberately heat treated so as to yield the preferred tastes and textures.

Experimental Techniques for Studying Enzyme Inactivation
There are different research methods that are employed by the scientists in examining enzyme inactivation and also for the development of inhibitors for use in medicine and other industries. These include:
- Spectrophotometry: Measures enzyme activity before and after exposure to inhibitors.
- X-ray Crystallography: Determines the structural changes in enzymes upon inactivation.
- Molecular Docking: This imitates the behavior of inhibitors in relation to enzymes at the molecular level.
Заключение
Enzyme inactivation is a very significant process that is in medicine, industry as well as in our daily lives. From enzyme inhibition mechanisms или irreversible enzyme inhibition , the regulation of the enzymes is very important in health, biotechnology and food processing.
Knowledge of such processes assists scientists in formulating better drugs, food preservation techniques, and even fighting diseases. The next time you swallow a pill or eat canned food, you will realize that enzyme inactivation is at play! For more detail, visit our blog section!
Часто задаваемые вопросы
- What is enzyme inactivation?
Enzyme inactivation on the other hand is the reduction of enzyme activity since its structure has changed and thus cannot catalyze reactions.
- How does irreversible enzyme inhibition work?
It inactivates an enzyme through other mechanisms, which are usually covalent modifications to the enzyme’s active site and renders it non-functional.
- What are common causes of enzyme inactivation?
Enzymes can be inactivated by temperature changes, changes in pH levels, presence of chemicals and Genetic mutations.
- Why is enzyme inactivation important in medicine?
It plays a significant role in drug development through developing toxins which are reactive to dangerous enzymes for instance bacteria or disease causing metabolic ones.
- How does the fact that enzymes can be inhibited from their action in the preservation of food?
Heat and chemical preservatives kill enzymes that cause spoilage and increase the time for which foods can be stored.
