That’s why understanding where are proteins synthesized is pivotal for progressing biomedical science
In this article, we will explore the cellular mechanisms of protein synthesis and their functions. We will also look into how bioreactors and fermenters play a big role in protein production in biotechnological processes. Let’s get to it…
The Basics of Protein Synthesis
Protein synthesis is a complex biological process where cells make proteins, the macromolecules that do almost everything in the cell, from enzymatic activity and structure to signaling and immune responses. Protein synthesis is based on the genetic information in DNA. This process has two main stages:
- Stage 1 – Transcription: DNA first gets converted into messenger RNA (mRNA) through transcription. It happens in the nucleus in eukaryotes & in prokaryotes in the cytoplasm. RNA polymerase recognises the DNA promoter, pulls the DNA out and makes mRNA. The mRNA gets edited and sent to the cytoplasm for translation.
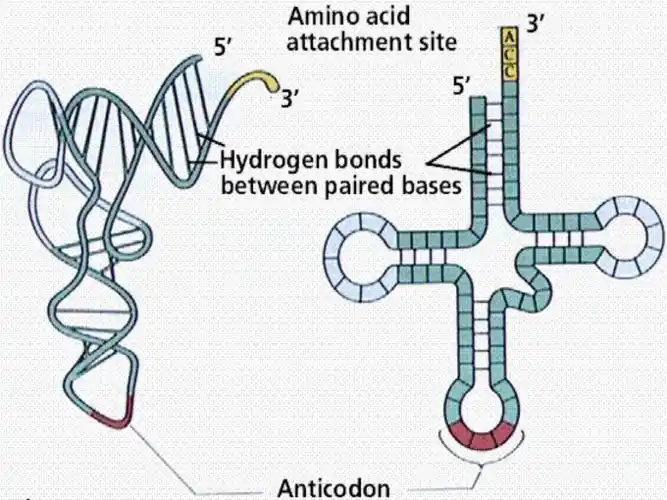
- Stage 2 – Translation: Translation takes place in the cytoplasm where proteins are assemble. Ribosomes read mRNA codon by codon for amino acid. Transfer RNA (tRNA) transports the correct amino acids which get bound together into a polypeptide chain and rolled into a functional protein.
Where Are Proteins Synthesized in the Cell?
Protein synthesis occurs in several key locations within the cell. These include the ribosome, rough endoplasmic reticulum (ER) and the cytoplasm. All of these cellular structures have unique functions in synthesising and tabling proteins, and thus give information on how cells can generate and control such molecules.
1). Ribosomes
Ribosomes are responsible for protein synthesis in both prokaryotic and eukaryotic cells. They have two main parts, the large subunit and the small subunit. These subunits are present as separate structures when the ribosome is not actively functioning, but combine during translation, which is the process of protein synthesis when amino acids join together to form polypeptides.
- Prokaryotic Ribosomes:In prokaryotic cells (bacteria) ribosomes float freely in the cytoplasm so they can quickly respond to the cell’s protein needs.
- Eukaryotic Ribosomes: Eukaryotic ribosomes may be floating free in the cytoplasm or attached to the rough ER. If attached to the ER, they are involved in the production of membrane bound or secreted proteins.

The Basics of Proteins Synthesized
2). Rough Endoplasmic Reticulum (ER)
The rough ER has ribosomes wrapped around its cytoplasmic surface, so it’s where proteins are produced for secretion or incorporation into cell membranes. Then, the proteins produced in the rough ER are transported to the Golgi machine, where they are purified and processed.
- Membrane Proteins: Produced in the rough ER, these are involved in keeping the cell together and in communicating with the outside world.
- Secretory Proteins: Proteins called secretory proteins (hormones, enzymes) are produced in rough ER and released from the cell.
The rough ER is the protein production centre, where proteins are synthesized and first folded into shape for use in the organism.
3). Cytoplasm
Free ribosomes in the cytoplasm manufacture proteins that work within the cell, like enzymes that carry out metabolic reactions. These proteins are internal to the cell and aren’t released from the cell.
Protein function in the cytoplasm:
- Enzymes
- Structural proteins
- Chaperone proteins
The cytoplasm is thus essential for the production of proteins directly sustaining the cell’s internal processes.
How Protein synthesized in Biotechnology?
In biotechnology, the production of proteins in large quantities is crucial for a wide range of industries, including pharmaceuticals, agriculture, and food production. And one of the most common way of making proteins in large quantities is through recombinant DNA technology. This is by introducing foreign DNA into cells to produce proteins that the host cell doesn’t make.
Recombinant Protein Production? What is it?
Recombinant protein production is a multi-step process which involves:
- Gene Cloning: First you need to find the gene that codes for the protein you want to produce. This gene is then inserted into a plasmid, a small circular piece of DNA that can replicate independently of the host cell’s chromosomes. This plasmid is the vehicle to carry the gene into the host cell.
- Transformation:Once the gene is cloned into the plasmid, the plasmid is introduced into a host cell. Host cells can be bacteria (like E. coli), yeast or mammalian cells. Once inside the host cell the gene is expressed and the cell starts producing the protein specified by the inserted gene.
- Expression Systems: The host cell – bacterial, yeast or mammalian – depends on the protein. For simple proteins bacterial systems are used, for proteins that require post-translational modifications like glycosylation (addition of sugar molecules to the protein) yeast or mammalian cells are used.
Fermentation and Protein Synthesis
Fermentation is a biological process where cells convert nutrients into energy, it’s a key part of biotechnology especially in large scale protein production. It provides controlled environment for cells to grow and synthesize proteins, that’s why it’s important in pharmaceuticals, food and industrial enzymes.
1). Microbial Fermentation
Microbial fermentation uses bacteria, fungi or yeast to produce proteins, often with genetic engineering.
- Bacterial Fermentation: Escherichia coli is a popular choice for its fast growth and high protein expression. It’s ideal for producing recombinant proteins where foreign genes are introduced. E. coli systems are cost effective and scalable.
- Yeast Fermentation: Yeast, especially Saccharomyces cerevisiae, is chosen for its ability to do post-translational modifications such as glycosylation. These modifications are important for therapeutic protein stability and functionality. Yeast systems are used in pharmaceuticals, biofuels and food industries.
2). Animal Cell Fermentation
Some proteins are complex, especially therapeutic ones, require animal cell fermentation. Mammalian cells such as Chinese Hamster Ovary (CHO) cells are ideal for producing high quality proteins like monoclonal antibodies and therapeutic enzymes. CHO cells do complex modifications and produce proteins that are very close to human proteins, important for medical treatments. In both microbial and animal cell fermentation, bioreactors are important to optimize conditions to maximize protein yield and minimize by-products, to ensure efficiency and scalability.
Bioreactors Role in Protein Synthesis
Bioreactors are the key to protein synthesis optimization. They offer a suitable environment where one can manipulate pH, temperature, oxygen and nutrient concentrations. These are the variables to be optimized for target protein and controlled for the by-products. Therefore, by providing the appropriate conditions for microbial or mammalian cells—bioreactors enhance protein yield and high quality proteins in large quantities.
And one of the important components of bioreactor technology is the indication and controlling of different factors that influence protein formation. Bioreactors in operation today have sophisticated control systems by which the fermentation or cell culture parameters can be made very precise. Here are some of the control systems used in bioreactors:
- pH Control:pH of the fermentation or culture medium has a big impact on the enzymes and microbial cells. A stable pH ensures the biochemical reactions involved in protein synthesis occurs efficiently. Automated pH control adds acid or base to maintain the optimal pH for growth and protein production.
- Temperature Control: Temperature is important for protein expression. Each organism or cell type has an optimal temperature range for metabolic activity. Bioreactors with temperature sensors and heating or cooling system maintains the temperature within this range to prevent protein denaturation and maximize production.
- Dissolved Oxygen Monitoring: Oxygen is crucial for aerobic organisms especially in processes where cell growth and protein synthesis depends on aerobic metabolism. The bioreactors have dissolved oxygen probes that enable continuous measurement of oxygen concentrations in order to meet the requirements for microbial or mammalian cell growth. Automated control system controls the aeration rates in order to regulate the supply and demand of oxygen which interferes with the cell functioning.
What Bioreactors are used in Protein Synthesis?
Depending on the protein, scale and requirements, different bioreactors are used in protein synthesis. Below are the most common types, their features, advantages and challenges:
1. Batch Bioreactors
Batch bioreactors are a simple solution for protein synthesis, especially for processes that don’t require continuous nutrient supply. They are used for small scale or pilot production.
Features:
- Operate in a closed system with a fixed volume of nutrients added at the start.
- Monitors and controls pH, oxygen and temperature throughout the process.
- Entire culture is harvested at the end of the production cycle.
Batch bioreactors are cost effective and easy to use, good for small scale or research purposes. But not efficient for large scale production as nutrient depletion and by-product accumulation over time can limit cell growth and protein yield.
2. Continuous Bioreactors
Another type of bioreactor is the continuous bioreactor intended for large-scale protein production. This kind of bioreactors is most suitable where it is necessary to maintain steady production over long periods of time.
Key Features:
- Fresh nutrients are continuously fed into the reactor while the culture is simultaneously harvested.
- There are advanced monitoring devices applied to control the nutrient and waste level.
- Advanced monitoring systems are integrated to maintain nutrient and waste balance.
Continuous systems proved to have high productivity and the protein produced is of a uniform quality, characteristics that makes it suitable for large scale production. But they need sophisticated control and are vulnerable to changes in operating conditions, which influences yield and product quality.
3. Fed-Batch Bioreactors
Fed-batch bioreactors blend the benefits of batch and continuous systems, making them versatile for producing high-value proteins like monoclonal antibodies and enzymes.
Key Features:
- Fed-batch bioreactors are a mix of the batch and the continuous processes which are useful in the production of valuable proteins such as monoclonal antibodies and enzymes.
- Prevents nutrient depletion and by-product accumulation that can inhibit cell growth.
- Enables precise control over feeding strategies to optimize protein production.
Due to their flexibility and the high yields that they provide, they can be used in laboratories as well as in industries. Yet, they require strict control of measurements, feeding frequency, and portion sizes which may lead to increased operationalication intricacy.

4. Air-Lift Bioreactors
Air-lift bioreactors are used for protein production from microbial or mammalian cells when low shear stress rates are required. They use gas flow for mixing not mechanical agitation.
Key Features:
- Use air or gas injection to create internal circulation and oxygen transfer.
- Gentle mixing for sensitive cell lines or proteins.
- Energy efficient with minimal mechanical parts.
Air-lift bioreactors are great for applications that require efficient oxygenation and low cell stress like mammalian cell culture for biopharmaceuticals. But gas flow for mixing limits their use to certain types of cells or proteins and scaling up requires careful design consideration.

5. Stirred Tank Bioreactors
Stirred tank bioreactors are the most used systems for protein production, they offer great flexibility and control over the production conditions.
Key Features:
- Have a mechanically driven impeller for uniform mixing of nutrients, gases and cells.
- Allow precise control of environmental parameters like temperature, pH and dissolved oxygen.
- Compatible with all scales from laboratory to industrial production.
These bioreactors are versatile, suitable for microbial and mammalian cell cultures. They maintain optimal conditions for high protein yield and quality. But mechanical parts can introduce shear stress that can affect sensitive cell lines or proteins.
Conclusion
Protein synthesis is a critical cellular process. And by knowing more about its operation and increasing its production efficiency through better bioreactors—the food, pharmaceutical and other industries can effectively fulfill the global needs for proteins. BaiLun Biotechnology Co., Ltd is leading the way by developing new and efficient bioreactors that fully address the current deficiencies of the old models.
BaiLun Biotechnology Co., Ltd bioreactors are functional, inexpensive, and dependable. Whether it is a lab-scale system or industrial equipment, BaiLun offers technological solutions and professional consulting services to ensure your success. Get in touch with BaiLun today and find out how we can enhance your biotechnological processes.